Background:
T cell lymphoma (TCL) is a group of highly heterogeneous aggressive non-Hodgkin lymphomas with different pathogenesis and clinical prognosis. Despite the survival benefits of anthracycline-based chemotherapy bridging autologous stem cell transplantation (ASCT), 40% to 50% of TCL patients fail to respond to treatment and relapse or die within a short period of time. Chidamide is an oral selective histone deacetylase inhibitor (HDACi), interferes with epigenetic regulation by deacetylating nuclear transcription factors, affects proliferation and differentiation of tumor cells, and regulates cytokines in the tumor microenvironment. Chidamide combined with chemotherapy has promising clinical applications in ASCT.
Aims:
This prospective Phase II trial aims to evaluate the efficacy and safety of chidamide in combination with carmustine, etoposide, cytarabine, and melphalan (Chi-BEAM) conditioning regimen in ASCT of TCL patients received CR/PR to first-line chemotherapy.
Method:
In this study, eligible patients with TCL (except IPI 0-1 ALK+ anaplastic large cell lymphoma) aged 18-65 years were enrolled. All patients received Chi-BEAM conditioning regimen before ASCT. 30mg of chidamide was administered twice a week for at least 2 years after ASCT.The primary end point was 2-year progression-free survival (PFS) rate, and the secondary end points were 2-year overall survival (OS) rate and safety evaluation.
Results:
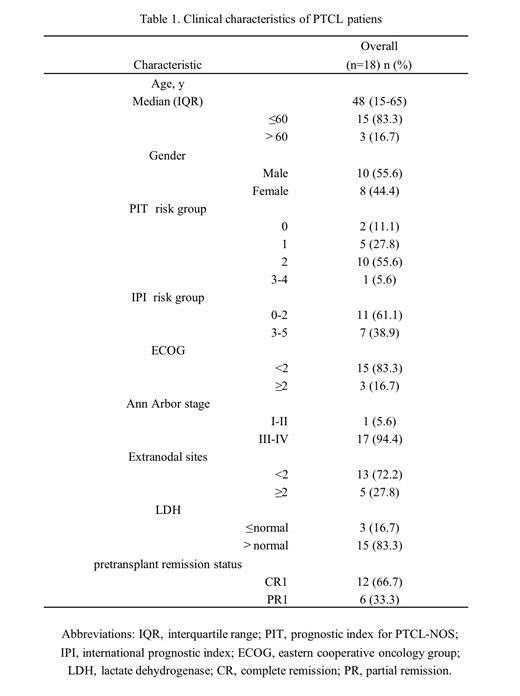
At the time of data cut off, a total of 18 patients were enrolled, with a median age of 48 (15-65) years. The 1-year PFS rate and OS rate after ASCT were 90.0% and 100.0%, respectively. The median implantation time of neutrophils was 10 days (9-13 days), and the median implantation time of platelets was 17 days (11-25 days). Most of the adverse reactions (AEs) were tolerable, including controlled grain deficiency with fever, electrolyte disturbance, and abnormal liver function.
Conclusion:
Chidamide combined with BEAM as a conditioning regimen for ASCT can significantly improve PFS and OS in TCL patients, and the AEs are controllable. The study is ongoing and further results will be continuously released.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal